Current Physician Treatment Preferences for Frontline Metastatic ccRCC

Arnab Basu, MBBS, MPH, FACP

Current Treatment Landscape in Clear Cell Renal Cell Carcinoma (ccRCC)
Treatment options for metastatic ccRCC have expanded significantly in the past few years. Single-agent VEGF receptor antagonists, such as sunitinib (Sutent), were the historical standard of care and were characterized by a modest response rate (30%) and a median progression-free survival (mPFS) of approximately 1 year.1
Lately, combination therapies have improved these outcomes. Modern doublet regimens combine either VEGF inhibitors (cabozantinib [Cabometyx], axitinib [Inlyta], or lenvatinib [Lenvima]) or a CTLA-4 inhibitor (ipilimumab [Yervoy]) to a PD-1 inhibitor backbone of pembrolizumab (Keytruda) or nivolumab (Opdivo). Although all of these are potential options, it remains challenging to identify those who may potentially optimally benefit from either of these strategies.
In 2018, ipilimumab plus nivolumab became the first approved frontline combination regimen for RCC, with an indication for patients with intermediate- to poor-risk disease based on International Metastatic RCC Database Consortium criteria. In the CheckMate 214 study (NCT02231749), combination therapy improved the mPFS from 8.4 months to 11.6 months.2 Although the objective response rate (ORR) was only 42%, a substantial group of patients experienced prolonged disease control, and at 8 years of follow-up, 12% of patients remained in radiographic complete response (CR), including 12.8% with favorable-risk disease. Further, the median duration of response was 82.8 months with the combination vs 19.8 months compared with sunitinib alone.3
In contrast to doublet immunotherapy, VEGF inhibitor–based combinations are characterized by higher response rates and rapid disease control. Pembrolizumab with axitinib, which was the first frontline VEGF-based combination approved in 2019, improved mPFS to 15.7 months vs 11.1 months and almost doubled the ORR (60.6% vs 39.6%) when compared with sunitinib.4
This was followed by cabozantinib and nivolumab in 2021, similarly showing a strong improvement in mPFS of 16.4 months vs 8.3 months and an ORR in 55.7% of patients compared with 27.1% with sunitinib in the CheckMate 9ER study (NCT03141177).5 Pembrolizumab with lenvatinib was the latest regimen to be approved, with an mPFS of 23.9 months vs 9.2 months and an ORR of 71.3% compared with 36.7% with sunitinib in the CLEAR study (NCT02811861).6
Table. Efficacy of Doublet Regimens in the Intention-to-Treat ccRCC Population3-6, a
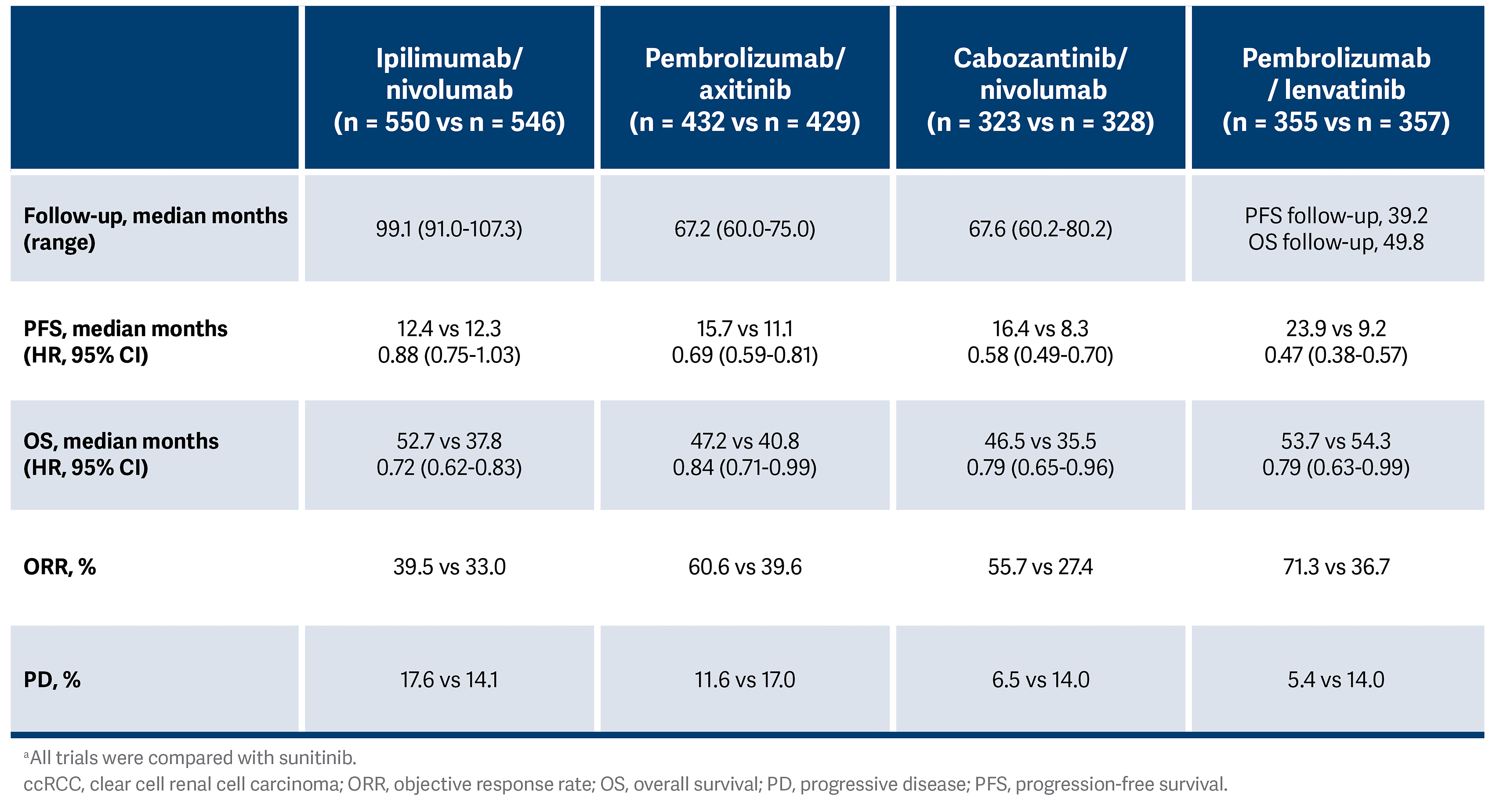
The similarity of these therapeutic agents has led to challenges in selecting the most appropriate treatment plan for patients. The Table summarizes the key efficacy statistics from these studies as of 2025. Adverse event profiles also differ among these regimens, with a higher rate of steroid use for immune adverse events with combination immunotherapy, but also a much higher rate of hypertension, fatigue, hand-foot syndrome, and other VEGF-related adverse events.
To understand current practice patterns in these settings, we compiled responses from poll-based questions obtained from Targeted Oncology’s Case-Based Roundtable discussions, which provide a forum for the broad oncology community to discuss important clinical management issues.
Case and Poll Question Results
Case Summary
- A 65-year-old man presented with back pain for the past 6 months and hematuria for 1 week.
- Laboratory results: hemoglobin, 11.4 g/dL; lactate dehydrogenase, 980 U/L; all others within normal limits
- CT scan of chest/abdomen/pelvis: multiple mediastinal and hilar nodes, deposits in left lower lobe of lung, enlarged axillary nodes, and enhancing mass in left renal parenchyma with renal vein infiltration; lytic destruction of L4 and L5 vertebrae, left superior pubic ramus, and right ischium
- Biopsy of renal mass and bone biopsy confirmed metastatic ccRCC.
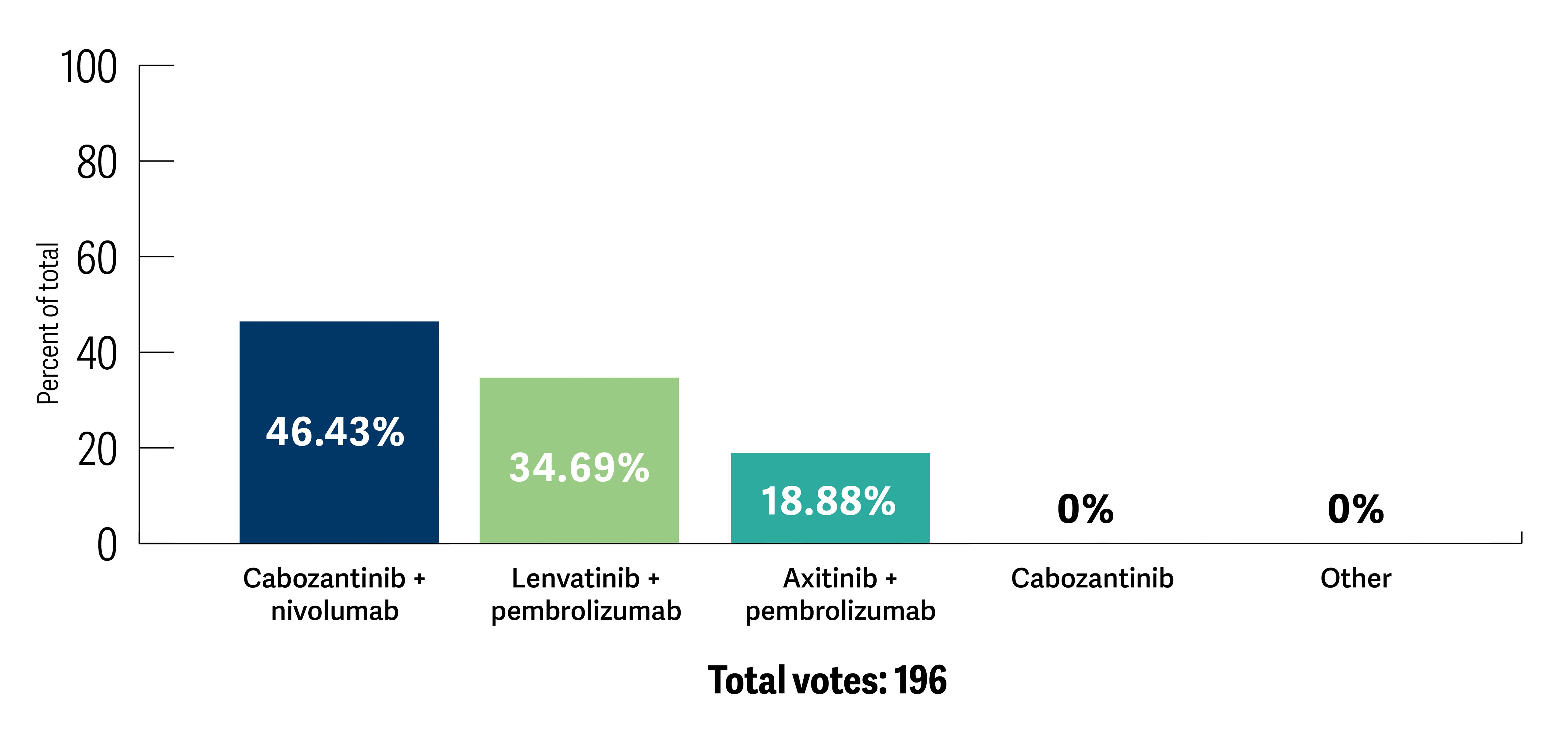
When considering an intermediate or poor-risk patient who has newly diagnosed high-volume disease with pulmonary and bony involvement, most participants (64.6%) selected a combination approach with a VEGF tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) combination. The combination of cabozantinib and nivolumab was the preferred regimen (46.4%), followed by lenvatinib and pembrolizumab (34.7%) and axitinib with pembrolizumab (18.9%) (Poll 1).
Poll 1. If a decision were made to initiate a TKI-ICI regimen, which first-line regimen would you most likely choose for this patient?
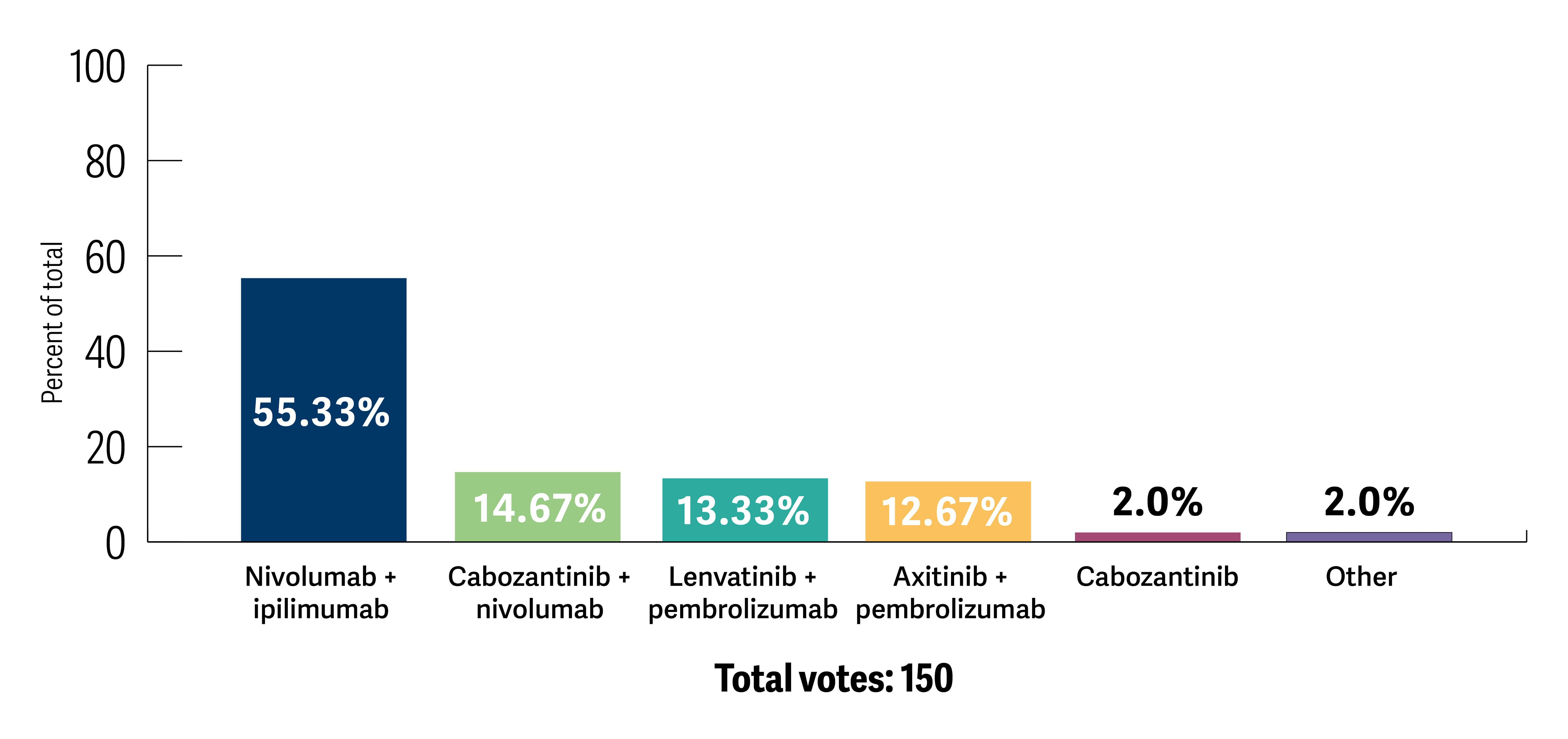
Oncologists preferred doublet checkpoint inhibition with ipilimumab and nivolumab when patients were asymptomatic with limited disease volume (55.3%), followed by cabozantinib and nivolumab (14.8%) as the preferred TKI/immunotherapy combination (Poll 2). The key drivers of treatment decisions appeared to be disease volume, as a marker of aggressiveness, in addition to sites of metastatic involvement and the International Metastatic RCC Database Consortium risk group.
Poll 2. Which first-line therapy are you likely to recommend if your patient was asymptomatic with metastatic disease limited to the liver or lung (ie, no bone involvement)?
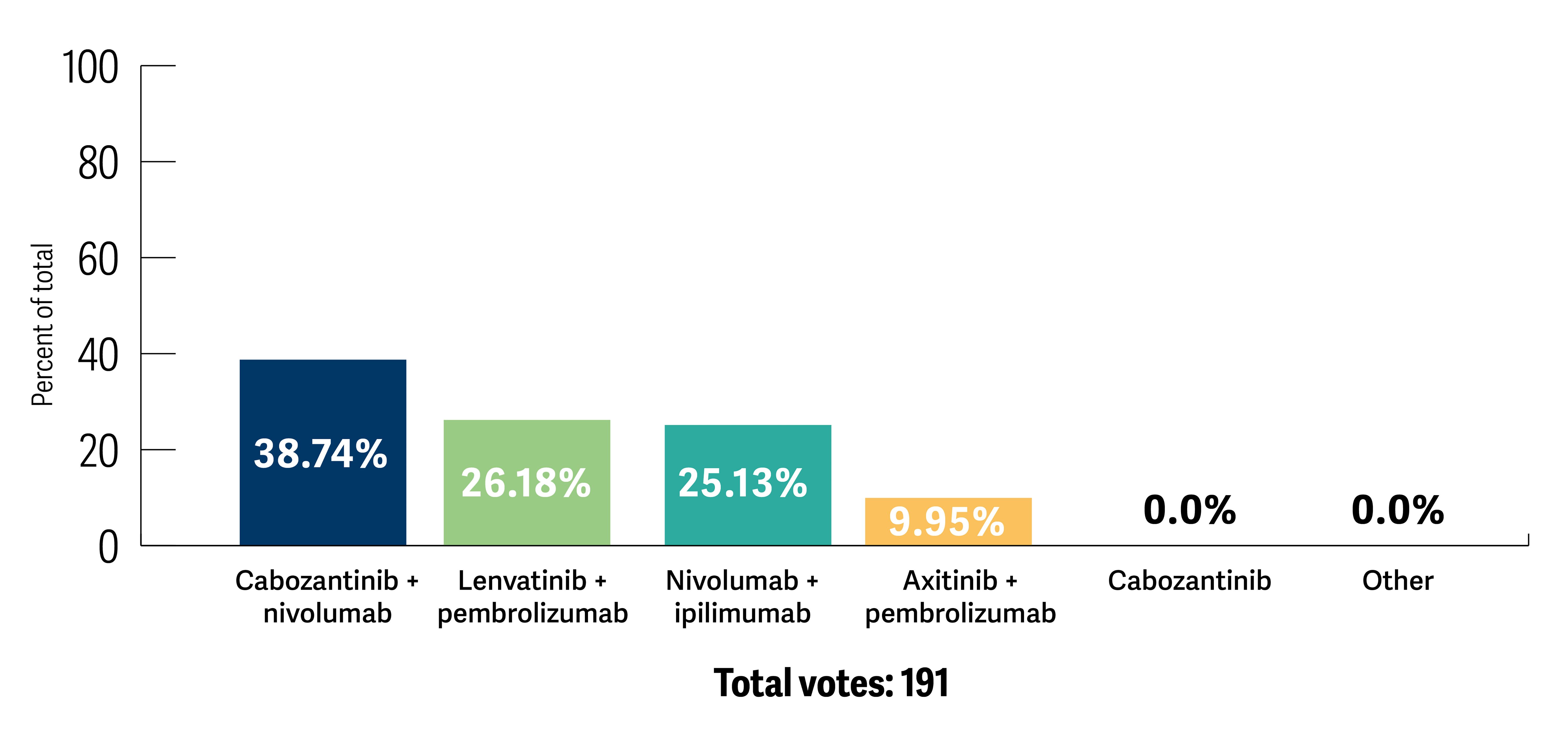
After reviewing the current therapeutic landscape and evidence from the clinical trials, participants were asked to re-evaluate their treatment choice. All participants chose a combination strategy, among which cabozantinib and nivolumab was the preferred option (Poll 3).
Poll 3. After discussion of this case and related trials, what first-line therapy would you recommend?
Conclusions
Considering the caveat of heterogeneity of populations in these studies, this approach is supported by current evidence. In the CheckMate 214 study, the ORR was only modestly improved; 18% of patients had progressive disease as the best response, which can be challenging to manage in cases with higher volume disease. In contrast, using a VEGF plus immunotherapy combination such as cabozantinib with nivolumab or lenvatinib with pembrolizumab can significantly lower the primary progression rates (7% and 5%, respectively). Continued indefinite therapy with TKIs is also associated with unique quality-of-life challenges, and patients who would like longer treatment-free intervals may benefit from an immunotherapy-only approach. Reassuringly, all the approaches appear to have robust median overall survival, in the 47- to 54-month range, proving that despite the challenges, the outcomes for patients with metastatic RCC have remarkably improved.
References:
1. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-124. doi:10.1056/NEJMoa065044
2. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi:10.1056/NEJMoa1712126
3. Tannir NM, Albigès L, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 8-year follow-up results of efficacy and safety from the phase III CheckMate 214 trial. Ann Oncol. 2024;35(11):1026-1038. doi:10.1016/j.annonc.2024.07.727
4. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced clear cell renal cell carcinoma: 5-year survival and biomarker analyses of the phase 3 KEYNOTE-426 trial. Nat Med. Published online August 1, 2025. doi:10.1038/s41591-025-03867-5
5. Motzer RJ, Escudier B, Burotto M, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for previously untreated advanced renal cell carcinoma (aRCC): Final follow-up results from the CheckMate 9ER trial. J Clin Oncol. 2025;43(suppl 5):439. 10.1200/JCO.2025.43.5_suppl.439
6. Motzer RJ, Porta C, Eto M, et al. Lenvatinib plus pembrolizumab versus sunitinib in first-line treatment of advanced renal cell carcinoma: final prespecified overall survival analysis of CLEAR, a phase III study. J Clin Oncol. 2024;42(11):1222-1228. doi:10.1200/JCO.23.01569
link